Previously, OUR GROUP was FOCUSED ON TWO MAIN PROBLEMS.
• how do worms respond rapidly to insults and what can we uncover by looking at rapid responses?
• can we use our unique platforms in C.elegans to identify new drugs to treat the 1.5 billion humans infected with parasites?
Drug screens
One of the beauties of genomics is it leads us into unexpected and unexplored directions. To complement our systematic genetics, we developed a new imaging platform that lets us measure the precise effect any drugs has on worm movement. The resolution and throughput are unique: we can measure the movement of 2000 worms simultaneously and measure their movement every 0.5s. This lets us do two key things:
1. Measure precisely how drugs affect worm movement and how worms respond to those acute insults. We can add drugs to worms and watch how their movement changes minute by minute. Capturing these acute drug responses has given very unexpected results: we find that worms show very complex responses to ~50% of all drugs we have tested (see Fig1). This has led to insights into how neurotransmitter signalling pathways talk to eachother in whole animals as well as the discovery of new metabolic pathways. We currently have multiple academic and industry collaborations to use this unique platform for key drug screens.
Figure 1: A diversity of movement responses when worms are acutely treated with various drugs and physiological stresses. For more information, see our paper published here
2. Screen for mutants with altered drug responses. The real power of our imaging platform is that we can identify individual worms with altered drug responses at high throughput at and high resolution — subtle changes to responses can be picked up accurately and fast. This means that we can do very rapid screens for mutants with altered drug responses — this identifies drug targets (Fig 2).
Thus far, in two pilot screens of our technology, we have identified numerous chemically mutagenized worms that show unique responses. In the first screen (Fig 3) we have found several strains that show greater resistance to the acute toxicity of the anti-parasitic drug levamisole. In the second pilot, we were able to isolate mutants that failed to recover from the hypoxia-simulating condition of cyanide and 2-deoxyglucose poisoning.
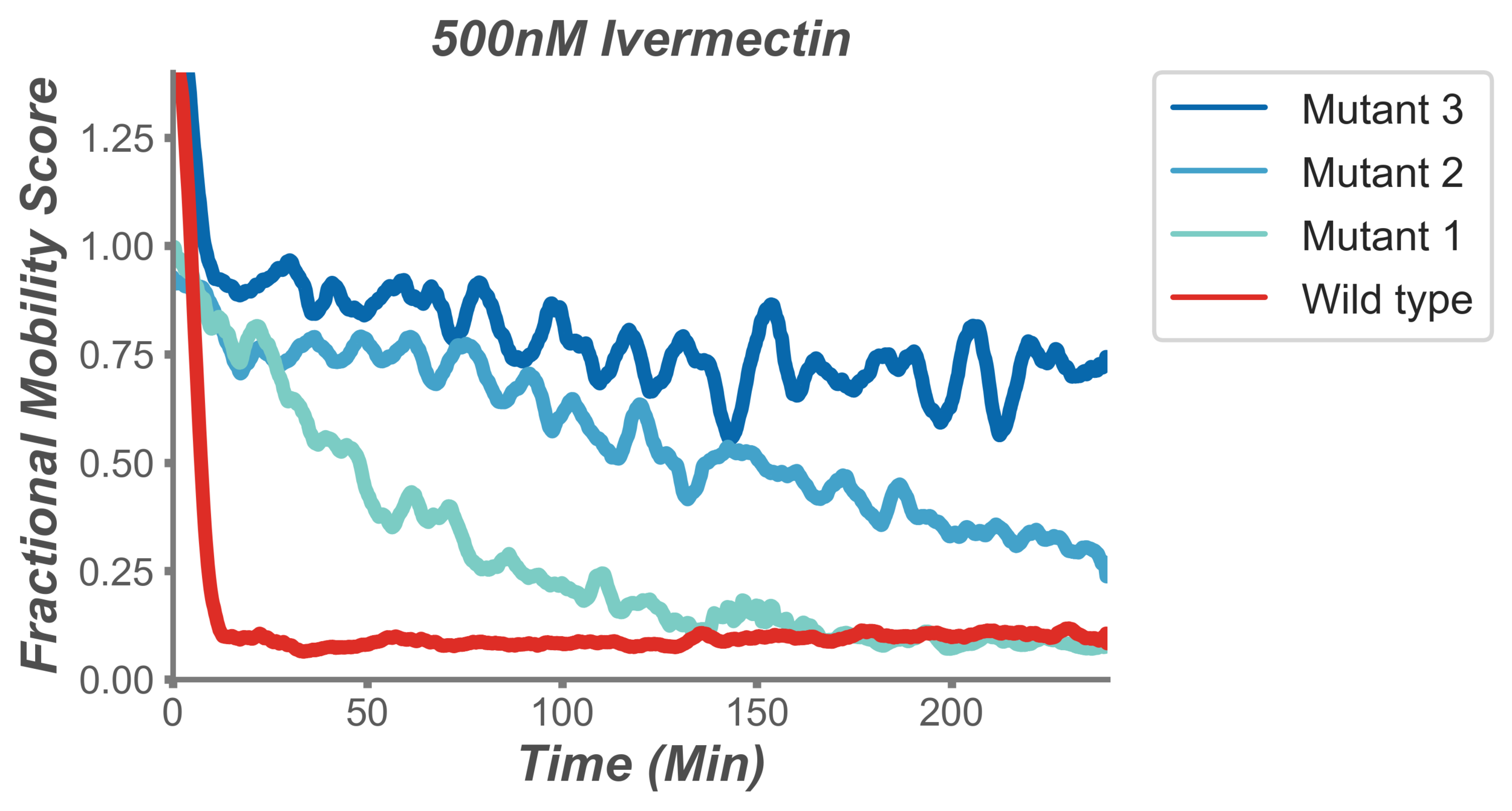
Figure 3: Identification of mutants resistant to the anthelmintic drug levamisole. Individual lines represent the thrashing motion of a population of worms in liquid over time. The behaviour of a normal population of worms (red) is to rapidly cease moving, while three populations of mutant worms (blue) show varying levels of resistance.
Figure 4: Identification of mutants that fail to recover from cyanide and glycolysis poisoning. As with the ivermectin resistance screen, we are able to find several mutants (blue) that show a different levels of recovery compared to the population of normal worms. This is a far more challenging screen and speaks to the power of our new imaging technology platform.
A unique platform to identify new anthelmintic drugs affecting nematode metabolism
Parasitic helminths like Ascaris, hookworm, and whipworm affect almost a quarter of the world’s population. Currently, while excellent drugs are available, resistance is rising — to tackle this we need new classes of drugs. We are trying to find these by targeting the unique metabolic pathways used by parasites when they are inside their hosts. C.elegans is the only model that uses these pathways and we have used our drug screening platform to establish clean assays for drugs that specifically target them. This project is extremely exciting — it combines high throughput drug screens with basic genetics and metabolomics to understand how parasites adapt to the anaerobic environment in their hosts and to identify key new generation therapies for this major global challenge. It’s rare to have the chance to affect the health of over a billion people and we have partnerships with academics, NGOs and pharma to drive this project.